Microtek Assembly Services
Supply Chain Management: From process design through production, we offer supply chain management that creates value for customers. We deliver a complete manufacturing solution for our clients: on time and high yield.

Supply Chain Management: From process design through production, we offer supply chain management that creates value for customers. We deliver a complete manufacturing solution for our clients: on time and high yield.
Testing
Prototype Form Factor
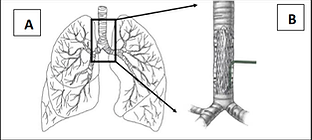
Figure. (A) Lung and (B) implantation of μIT Catheter in the trachea.
References
1. Bousquet, J., Dahl, R. & Khaltaev, N. Global alliance against chronic respiratory diseases. Allergy 62, 216-223 (2007).
2. Mestre-Ferrandiz, J., Sussex, J. & Towse, A. THE R&D COST OF A NEW MEDICINE. London, Office of Health Economics (2012).
3. Barnes, P.J., et al. Barriers to new drug development in respiratory disease. The European respiratory journal 45, 1197-1207 (2015).
Respiratory disease is a global pandemic, affecting over 1 billion people worldwide.[1] With the rising prevalence of pulmonary disorders, such as asthma and chronic obstructive pulmonary disease, there is an increasing need for new medications. In the pharmaceutical industry, of the compounds being developed for key therapeutic areas, approximately 7-10% represent therapeutic compounds being developed for respiratory diseases.[2] However, the rate of market approval for respiratory drugs is only 3%, whereas drugs treating other health conditions, such as cancer and cardiovascular diseases, have a 6-14% chance of obtaining FDA approval.[3] Historically, poor pharmacokinetics, bioavailability, and toxicology were common causes for drug failure. While the industry has made advancements in the tools used to accurately predict these parameters and increase advancements to clinical trials, this has not been the case for inhaled formulations.
To address these limitations, Microtek is developing a new tool – the μIT Catheter – for use during the preclinical stages of drug development of inhaled therapeutics. The μIT Catheter, a self-expandable intratracheal lung delivery device, will nebulize drugs in the trachea and efficiently distribute the particles in the lungs when administered using single or multiple dosing strategies. The integration of this new tool will greatly improve the early stage drug development process for respiratory drugs and help advance therapeutics towards FDA approval.
Wire Bonding
Die Attach
Flip Chip and Bumping
Surface Mounts Components
Operations
We are compassionate innovators at heart, which drives us to share our creations with the world!
Tech News
Microtek’s wire bonding services provide customers with considerable scope and flexibility in product design. We work [...]
Read MoreTech News
Microtek partners with Mission Driven Finance for an impact-based financing for our current growth phase. [...]
Read More